Florida Blue Coverage on Colonoscopy With Family History
Colonoscopy Screening Amongst US Adults Aged 40 or Older With a Family History of Colorectal Cancer
Meng-Han Tsai, MHA; Sudha Xirasagar, PhD, MBBS; Yi-Jhen Li, PhD; Piet C. de Groen, MD
Suggested citation for this article: Tsai M, Xirasagar South, Li Y, de Groen PC. Colonoscopy Screening Among Usa Adults Aged 40 or Older With a Family History of Colorectal Cancer. Prev Chronic Dis 2015;12:140533. DOI: http://dx.doi.org/10.5888/pcd12.140533external icon.
PEER REVIEWED
PEER REVIEWED
- Abstruse
- Introduction
- Methods
- Results
- Discussion
- Acknowledgments
- Author Information
- References
- Tables
- Appendix
Abstract
Introduction
Colonoscopy screening reduces colorectal cancer (CRC) incidence and bloodshed. CRC screening is recommended at age 50 for average-risk people. Screening of first-degree relatives of CRC patients is recommended to begin at age 40 or 10 years earlier the historic period at diagnosis of the youngest relative diagnosed with CRC. CRC incidence has increased recently among younger Americans while it has declined among older Americans. The objective of this report was to determine whether first-caste relatives of CRC patients are existence screened co-ordinate to recommended guidelines.
Methods
We studied colonoscopy screening rates amid the United states population reporting a CRC family history using 2005 and 2010 National Health Interview Survey data.
Results
Of 26,064 study-eligible respondents, 2,470 reported a CRC family history; of those with a family history, 45.6% had a colonoscopy (25.2% in 2005 and 65.8% 2010). The colonoscopy rate among first-degree relatives aged 40 to 49 in 2010 (38.3%) was well-nigh half that of first-caste relatives aged 50 or older (69.7%). Commencement-caste relatives were nearly twice as likely as nonfirst-degree relatives to accept a colonoscopy (adjusted odds ratio [AOR], 1.7; 95% confidence interval, 1.5–ane.9), but those anile 40 to 49 were less likely to accept a colonoscopy than those in older historic period groups (AOR, two.half-dozen for historic period l–64; AOR, 3.vi for historic period ≥65). Interactions with age, insurance, and race/ethnicity were not significant. Having health insurance tripled the likelihood of screening.
Conclusion
Despite a 5-fold increase in colonoscopy screening rates since 2005, rates amidst commencement-degree relatives younger than the conventional screening age have lagged. Screening promotion targeted to this group may halt the recent rising tendency of CRC among younger Americans.
Tiptop
Introduction
Colorectal cancer (CRC) is the 2d leading cancer in the United States; 132,700 new cases and 49,700 deaths are expected in 2015 (1–iii). The 4.3% almanac decline in incidence among adults aged 50 or older is marred past the concurrent 1.viii% annual increase among adults younger than 50; an increase in CRC incidence of 28% to 46% is anticipated for this younger historic period group by 2030 (1,iii). Younger CRC patients typically receive a diagnosis of more avant-garde disease and have poorer survival rates than older CRC patients, and they account for half dozen.5% of total CRC deaths (2,4).
About thirty% of CRC patients report a family history of CRC: of those, 5% accept 1 of the well-characterized inherited syndromes (eg, Lynch syndrome, familial adenomatous polyposis), and the remaining 25% are starting time-degree relatives of sporadic (nonhereditary) CRC patients. A beginning-degree relative is a biological parent, sibling, or child of a CRC patient (five–7). First-degree relatives accept two to 3 times the risk of developing advanced adenomas and cancer than the general population. The risk increases equally the relative'due south age at diagnosis decreases and the number of relatives with CRC increases (8,nine). About 23% of CRC patients younger than 45 years written report a family history of CRC (10). Timely screening of first-degree relatives is therefore an important tool in decreasing rates of CRC.
Because colonoscopy allows for the removal of beneficial polyps that cause 75% to lxxx% of CRCs, colonoscopy screening tin reduce CRC incidence past 83% and CRC bloodshed by 89% (11–14). The American College of Gastroenterology recommends get-go-degree relatives of CRC patients who received their cancer diagnosis before age 60 to brainstorm colonoscopy screening at age 40 (13). The recent increase in CRC incidence among younger adults calls for greater attending to younger beginning-caste relatives (iii,iv). Studies on screening rates among outset-caste relatives are dated, are express to those aged 50 or older, or are unmarried-center studies (15,16). Systematic reviews found low rates of colonoscopy screening among commencement-degree relatives (31%–40%) even though most guidelines emphasize the importance of colonoscopy screening for this higher-gamble group (16). We conducted a population-based report of CRC screening among first-degree relatives younger (aged twoscore–49) than the conventional screening age of 50.
Top
Methods
Study population
Pooled information from the 2005 and 2010 National Wellness Interview Survey (NHIS) were used. The NHIS is an annual, cross-sectional, nationally representative household survey that collects information on CRC every 5 years through the department "Family History and Cancer Screening." The questions on cancer family history (Appendix) ask whether the respondent's biological parent, sibling, or child always had cancer and enquire nigh the cancer blazon. The questions on CRC screening type and timing of the nigh recent screening examination are consistent with the US Preventive Services Task Force (USPSTF) screening recommendations (12,13,17). Our written report sample consisted of all screening-eligible respondents in the 2 surveys (Figure): all respondents aged 50 or older and all respondents aged 40 to 49 who reported a family history of CRC. Of the 36,575 respondents anile forty or older in the 2 surveys, we excluded x,511 respondents aged 40 to 49 who either reported not having a CRC family unit history (n = 3,517) or did non reply to the question (n = 6,994). Of 26,064 CRC screening–eligible respondents, 2,470 were first-caste relatives of CRC patients and 10,454 were nonfirst-degree relatives; nosotros were unable to determine the family history of xiii,140 respondents (aged 50 or older who did not respond to the family history question).
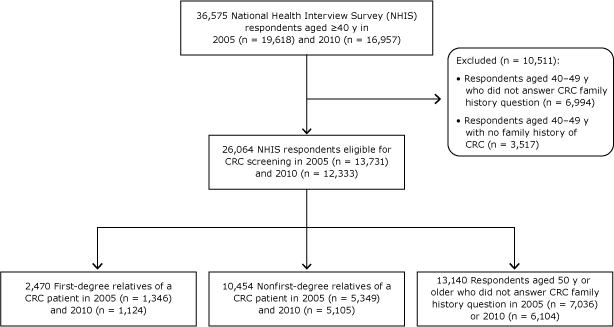
Figure. Study sample selected from respondents to the National Health Interview Surveys, 2005 and 2010. Abridgement: CRC, colorectal cancer. [A text version of this figure is available.]
Measures
Considering colonoscopy is the well-nigh effective screening tool to prevent CRC and may be peculiarly important for beginning-degree relatives (11,13), we analyzed colonoscopy screening and whatsoever CRC screening. Per USPSTF recommendations, CRC screening completion was divers every bit the following: colonoscopy in the previous 10 years, a fecal blood test (FOBT or FIT) in the by year, sigmoidoscopy in the previous 5 years, or computed tomography (CT) colonography in the previous 5 years (12,13). Our key dependent variables of involvement were colonoscopy completion (yes or no) and any CRC screening (yes or no). The key independent variables were family unit history of CRC (first-degree relative, nonfirst-degree relative, or no response to the question), age (forty–49 y, 50–64 y, or ≥65 y), race/ethnicity (white, African American, Hispanic, or other) and wellness insurance (private; public, including Medicaid and Medicare; or uninsured). Survey yr (2005 or 2010) was used to examine changes over fourth dimension. We controlled for marital condition and instruction level (18).
Statistical analysis
We used χtwo tests to examine differences between subgroups separately for 2005 and 2010. Weighted logistic regression analyses of the pooled data were used to model the likelihood of colonoscopy screening (vs no colonoscopy) and whatever CRC screening (vs no screening). We studied associations with CRC family history, wellness insurance, historic period, sex, and race/ethnicity, controlling for marital status and education. Interactions of family history with historic period, sex, race/ethnicity, and wellness insurance were tested. Nosotros conducted weighted regression analyses to business relationship for the stratification bureaucracy and nonresponse bias and used a 2-sided test of significance at the .01 level every bit recommended by the National Center for Health Statistics. SAS v9.3 (SAS Institute Inc) was used.
Top
Results
The pooled sample consisted of 26,064 respondents (thirteen,731 in 2005 and 12,333 in 2010). The colonoscopy rate was 49.1% in 2010, almost 3 times the rate of xvi.v% in 2005. Amidst get-go-caste relatives, 65.viii% completed colonoscopy in 2010 and 25.two% in 2005, compared with 57.0% in 2010 and 19.0% in 2005 among non-first-degree relatives and 38.5% in 2010 and 12.7% in 2005 among nonrespondents (Table ane). Use of flexible sigmoidoscopy, FOBT, and CT colonography was negligible. Although the colonoscopy rate tripled from 2005 to 2010 among starting time-degree relatives anile xl to 49 (Table 2), the charge per unit among this younger group in 2010 (38.3%) was well-nigh half the charge per unit (69.7%) among showtime-caste relatives aged 50 years or older (69.ane% amongst adults aged 50–64 and lxx.3% among those aged ≥65).
The likelihood of having a colonoscopy versus non having a colonoscopy was 5 times higher in 2010 than in 2005 subsequently adjustment for age, sex, race/ethnicity, wellness insurance, and other covariates (adjusted odds ratio [AOR], five.4; 95% conviction interval [CI], 5.0–5.8) (Table three). First-degree relatives were 70% more likely than nonfirst-degree relatives to have a colonoscopy (AOR, 1.vii; 95% CI, 1.five–1.9). Nonrespondents were well-nigh half as probable every bit nonfirst-degree relatives to have a colonoscopy. The likelihood of colonoscopy among starting time-degree relatives aged 40 to 49 was about one-third that of the older historic period groups (AOR, two.half-dozen for those aged 50–64; AOR, three.6 for those aged ≥65). Respondents who had private or public wellness insurance were three times as likely equally those who were not insured to have a colonoscopy (AOR, three.3 for private insurance; AOR, 3.4 for public insurance). African Americans and whites were equally probable to have a colonoscopy. Interactions of outset-degree relative status with historic period, wellness insurance, and race/ethnicity were not meaning. The results did not alter when data on all screening tests were combined.
Top
Give-and-take
The key finding of this written report is that first-degree relatives younger than the conventional screening age of 50 were less likely than adults aged 50 or older to accept had a colonoscopy. Although studies certificate screening rates among first-degree relatives of inherited CRC syndromes, few studies are available on get-go-degree relatives of sporadic CRC patients, fifty-fifty though they are the largest higher-risk subgroup in the population. Our study shows the need for increasing screening rates in this subgroup, particularly first-degree relatives younger than 50 because of the recent increase in CRC among American adults in this age group.
The low colonoscopy rate amidst younger outset-degree relatives (40%) observed in our study should be viewed in light of the multifold increment in colonoscopy rates between 2005 and 2010 (69.vii%) amid adults in the widely publicized screening historic period group. Studies of first-caste relatives of sporadic CRC patients either focused on first-degree relatives aged 50 or older or used mixed-age samples without distinguishing younger start-degree relatives. Our other study findings are also consistent with those of earlier studies (xix-22). Amongst the population aged fifty or older, colonoscopy rates were college amid first-degree relatives than amid nonfirst-degree relatives. A recent study using NHIS 2010 information on adults aged 50 or older reported colonoscopy rates of 72.3% among first-degree relatives and 53.5% among nonfirst-degree relatives, similar to our finding (19). A study based on NHIS 2000 information on adults aged 41 to 75 reported colonoscopy rates of 27.8% amid offset-degree relatives and 7.vii% among nonfirst-degree relatives (20). Only 1 population-based study using NHIS 2000 data on younger offset-caste relatives is available: although information technology did not distinguish amid screening types, it reported that 15.viii% of men and 8.9% of women anile 40 to 49 had a CRC screening test (22). One meta-analysis of 7 studies reported a pooled colonoscopy charge per unit of xl% among all commencement-degree relatives anile 40 or older; no study in the analysis included contempo data (23).
Our findings are also consistent with the findings of unmarried-eye studies. A exercise-based patient survey in 2004 showed a colonoscopy rate of 29.half-dozen% amid first-degree relatives younger than 50 and a rate of 76% among first-degree relatives aged l or older. Just 39% of first-degree relatives younger than 50 had ever been asked past their physician about a CRC family unit history, and virtually one-half (46%) believed that screening should begin at age fifty (24). Some other study reported that the lack of physician recommendation was the unmarried well-nigh of import reason that first-caste relatives younger than 50 years had non undergone colonoscopy screening (25). Lack of awareness among first-degree relatives of the need for early screening and lack of physician recommendation appear to be major reasons for the depression screening rates amid first-caste relatives younger than 50. Screening education may have a greater outcome among starting time-caste relatives because of their personal exposure to CRC through family members.
Consistent with prior studies, we found that having health insurance increased the likelihood of colonoscopy screening (19,23,26,27). Colonoscopy screening rates among Medicare enrollees increased after 2001, when colonoscopy coverage was launched, from a mean quarterly rate of 285 per 100,000 beneficiaries during 1992–1997 to one,919 per 100,000 beneficiaries during 2001–2002 (28). The Affordable Intendance Act (ACA) now requires first-dollar coverage of preventive services, including colonoscopy, a provision that was not in strength during the NHIS 2010 survey. Screening promotion amidst younger first-degree relatives in the ACA surroundings has a better chance of increasing screening rates than in the pre-ACA surround, although nosotros would expect some attenuation of effect because of the general tendency of younger adults non to avail themselves of preventive health services and because grandfathered wellness plans are not required to conform to ACA provisions (4).
Our written report has several limitations. Ane is response-rate bias: one-half of the sample did not reply the family history question, and they were systematically different from the half that did answer the question: they were half as probable equally nonfirst-degree relatives to have undergone colonoscopy screening. Another limitation is that information were self-reported (ie, information were non extracted from medical records), which may accept resulted in overestimation of screening rates (nineteen,29). Finally, imbalanced prison cell sizes of the family unit history variable (2,470 vs 10,454) may limit the accuracy of odds ratio estimates.
Despite these limitations, our study is of import in highlighting that showtime-degree relatives aged forty to 49 of CRC patients are an undertargeted (and potentially rewarding) grouping for focused promotion of CRC prevention. Screening promotion should target both physicians and patients: alerting primary care physicians to appoint younger patients in learning almost a potential CRC family unit history and educating CRC patients to alert their first-degree relatives to initiate screening discussions with their physicians. Our recent report of an 83% reduction in CRC incidence and an 89% reduction in CRC mortality after screening colonoscopies should boost enthusiasm for colonoscopy screening among both patients and physicians (xi). Coupled with the ACA provisions requiring coverage of screening procedures, such efforts can help arrest the increment in CRC amongst Americans younger than l years (1,10).
Meridian
Acknowledgments
This piece of work was partly supported past funding from the National Cancer Constitute under grant no. 1R15CA156098-01 (Sudha Xirasagar, principal investigator) and the Mayo Clinic.
Top
Author Information
Corresponding Author: Sudha Xirasagar, PhD, MBBS, Department of Health Services Policy and Management, Academy of Due south Carolina, Arnold School of Public Health, 915 Greene St, Room 352, Columbia, SC 29208. Phone: 803-576-6093. Email: sxirasagar@sc.edu.
Author Affiliations: Meng-Han Tsai, Yi-Jhen Li, University of South Carolina, Columbia, Southward Carolina; Piet C. de Groen, Mayo Clinic College of Medicine, Rochester, Minnesota.
Acme
References
- American Cancer Lodge. Cancer facts & figures 2015. Atlanta (GA): American Cancer Society; 2015.
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64(2):104–17. CrossRefexternal icon PubMedexternal icon
- Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg 2015;150(1):17–22. CrossRefexternal icon PubMedexternal icon
- 4. Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B, et al. Colorectal cancer in U.S. adults younger than l years of age, 1998–2001. Cancer 2006;107(5 Suppl):1153–61. CrossRefexternal icon PubMedexternal icon
- Grady WM. Genetic testing for high-risk colon cancer patients. Gastroenterology 2003;124(half-dozen):1574–94. CrossRefexternal icon PubMedexternal icon
- Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology 2010;138(6):2044–58. CrossRefexternal icon PubMedexternal icon
- Kerber RA, Neklason DW, Samowitz WS, Burt RW. Frequency of familial colon cancer and hereditary nonpolyposis colorectal cancer (Lynch syndrome) in a large population database. Fam Cancer 2005;four(three):239–44. CrossRefexternal icon PubMedexternal icon
- Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006;42(two):216–27. CrossRefexternal icon PubMedexternal icon
- Cottet V, Pariente A, Nalet B, Lafon J, Milan C, Olschwang S, et al. Colonoscopic screening of first-caste relatives of patients with big adenomas: increased risk of colorectal tumors. Gastroenterology 2007;133(four):1086–92. CrossRefexternal icon PubMedexternal icon
- Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective report of family history and the take chances of colorectal cancer. N Engl J Med 1994;331(25):1669–74. CrossRefexternal icon PubMedexternal icon
- Xirasagar South, Li YJ, Hurley TG, Tsai MH, Hardin JW, Hurley DM, et al. Colorectal cancer prevention by an optimized colonoscopy protocol in routine practice. Int J Cancer 2015;136(6):E731–42. PubMedexternal icon
- 1US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Chore Forcefulness recommendation statement. Ann Intern Med 2008;149(9):627–37. CrossRefexternal icon PubMedexternal icon
- Male monarch DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104(3):739–50. CrossRefexternal icon PubMedexternal icon
- Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer past colonoscopic polypectomy. N Engl J Med 1993;329(27):1977–81. CrossRefexternal icon PubMedexternal icon
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among immature men and women in the The states. Cancer Epidemiol Biomarkers Prev 2009;eighteen(6):1695–8. CrossRefexternal icon PubMedexternal icon
- Henrikson NB, Webber EM, Goddard KA, Scrol A, Piper M, Williams MS, et al. Family unit history and the natural history of colorectal cancer: systematic review. Genet Med 2015. [Epub ahead of impress]. PubMedexternal icon
- National Center for Health Statistics, Sectionalisation of Wellness Interview Statistics, Centers for Disease Control and Prevention. National Health Interview Survey public use data file 2010. Hyattsville (Md): National Center for Health Statistics; 2011.
- James TM, Greiner KA, Ellerbeck EF, Feng C, Ahluwalia JS. Disparities in colorectal cancer screening: a guideline-based analysis of adherence. Ethn Dis 2006;16(1):228–33. PubMedexternal icon
- Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test apply, including CT colonography, in the 2010 National Wellness Interview Survey. Cancer Epidemiol Biomarkers Prev 2012;21(6):895–904. CrossRefexternal icon PubMedexternal icon
- Longacre AV, Cramer LD, Gross CP. Screening colonoscopy utilize amidst individuals at higher colorectal cancer risk. J Clin Gastroenterol 2006;40(six):490–six. CrossRefexternal icon PubMedexternal icon
- Mack LA, Cook LS, Temple WJ, Carlson LE, Hilsden RJ, Paolucci EO. Colorectal cancer screening amid first-caste relatives of colorectal cancer patients: benefits and barriers. Ann Surg Oncol 2009;xvi(8):2092–100. CrossRefexternal icon PubMedexternal icon
- Shah 1000, Zhu K, Palmer RC, Wu H. Family history of cancer and utilization of prostate, colorectal and pare cancer screening tests in U.South. men. Prev Med 2007;44(5):459–64. CrossRefexternal icon PubMedexternal icon
- Ait Ouakrim D, Lockett T, Boussioutas A, Hopper JL, Jenkins MA. Screening participation for people at increased run a risk of colorectal cancer due to family unit history: a systematic review and meta-analysis. Fam Cancer 2013;12(3):459–72. CrossRefexternal icon PubMedexternal icon
- Fletcher RH, Lobb R, Bauer MR, Kemp JA, Palmer RC, Kleinman KP, et al. Screening patients with a family history of colorectal cancer. J Gen Intern Med 2007;22(4):508–thirteen. CrossRefexternal icon PubMedexternal icon
- Codori AM, Petersen GM, Miglioretti DL, Boyd P. Health beliefs and endoscopic screening for colorectal cancer: potential for cancer prevention. Prev Med 2001;33(2 Pt i):128–36. CrossRefexternal icon PubMedexternal icon
- Courtney RJ, Paul CL, Sanson-Fisher RW, Macrae FA, Carey ML, Attia J, et al. Individual- and provider-level factors associated with colorectal cancer screening in accordance with guideline recommendation: a community-level perspective across varying levels of risk. BMC Public Health 2013;13:248. CrossRefexternal icon PubMedexternal icon
- Matthews BA, Anderson RC, Nattinger AB. Colorectal cancer screening behavior and wellness insurance status (Usa). Cancer Causes Control 2005;16(half-dozen):735–42. CrossRefexternal icon PubMedexternal icon
- Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA 2006;296(23):2815–22. CrossRefexternal icon PubMedexternal icon
- Gordon NP, Hiatt RA, Lampert DI. Cyclopedia of self-reported information and medical record audit for half dozen cancer screening procedures. J Natl Cancer Inst 1993;85(7):566–70. CrossRefexternal icon PubMedexternal icon
Superlative
Tables
 Table 1. Colorectal Cancer (CRC) Screening–Eligible Adult Respondents Aged xl Years or Older, Classified By CRC Family Historya, National Health Interview Survey 2005 and 2010 (n = 26,064)
Table 1. Colorectal Cancer (CRC) Screening–Eligible Adult Respondents Aged xl Years or Older, Classified By CRC Family Historya, National Health Interview Survey 2005 and 2010 (n = 26,064)
| Characteristic | 2005 (due north = 13,731) | 2010 (n = 12,333) | ||||
|---|---|---|---|---|---|---|
| Take a Family History,% (n = 1,346) | No Family History,% (n = five,349) | No Response,% (n = vii,036) | Have a Family History,% (n = ane,124) | No Family History,% (n = 5,105) | No Response,% (n = 6,104) | |
| Sexb | ||||||
| Male | 37.1 | 39.four | 45.5 | 37.5 | forty.4 | 45.4 |
| Female person | 62.9 | 60.6 | 54.5 | 62.five | 59.6 | 54.6 |
| Ageb, y | ||||||
| twoscore–49 | 18.5 | 0 | 0 | 17.7 | 0 | 0 |
| fifty–64 | 38.1 | 54.6 | 54.5 | 41.3 | 54.6 | 55.3 |
| ≥65 | 43.iv | 45.4 | 45.v | 41.0 | 45.4 | 44.7 |
| Race/ethnicityb | ||||||
| White | 85.v | 87.0 | 74.seven | 83.1 | 83.half-dozen | 71.1 |
| African American | 7.9 | 7.1 | 12.9 | 9.0 | eight.7 | 13.4 |
| Hispanic | 4.one | 4.2 | 8.0 | 4.nine | 5.five | 10.2 |
| Other | 2.4 | 1.8 | four.v | 2.9 | 2.2 | v.3 |
| Insuranceb | ||||||
| Private | 44.9 | 43.8 | 41.nine | 46.iii | 41.2 | 40.0 |
| Public | 48.7 | 49.viii | 49.7 | 46.0 | 51.5 | 49.9 |
| Uninsured | 6.4 | 6.three | viii.0 | 7.iv | seven.1 | 9.8 |
| CRC screening typeb | ||||||
| Colonoscopy | 25.2 | 19.0 | 12.seven | 65.8 | 57.0 | 38.5 |
| Sigmoidoscopy | 1.iv | 2.7 | ane.three | 0.iv | 1.ii | 0.9 |
| FOBT | 0.5 | 0.5 | 0 | 1.7 | 3.2 | ii.6 |
| CT colonography | NA | NA | NA | 0.i | 0 | 0.one |
| No screening | 73.0 | 77.9 | 85.vii | 32.1 | 38.6 | 57.9 |
Abbreviations: CT, computed tomography; FOBT, fecal occult claret examination; NA, not asked in the 2005 survey.
a Family history defined as cocky-reporting as a first-caste relative (biological parent, sibling, or child) of a CRC patient.
b P < .001 for all tests of departure between family history, no family history, and no response groups.
 Table two. Colorectal Cancer (CRC) Screening Status by Age Grouping and CRC Family unit Historya, National Health Interview Survey 2005 and 2010 (n = 26,064)
Table two. Colorectal Cancer (CRC) Screening Status by Age Grouping and CRC Family unit Historya, National Health Interview Survey 2005 and 2010 (n = 26,064)
| Family History/Age Grouping | No.b | 2005 | 2010 | ||||
|---|---|---|---|---|---|---|---|
| Colonoscopy | FS/FOBT/CT–C | No Screening | Colonoscopy | FS/FOBT/CT–C | No Screening | ||
| Take a family history (due north = ii,470) | |||||||
| 40–49 y | 251/196 | 12.7 | ane.6 | 85.7 | 38.3 | 3.ane | 58.7 |
| 50–64 y | 524/470 | 21.four | 1.three | 77.3 | 69.i | 1.7 | 29.1 |
| ≥65 y | 571/458 | 32.0 | two.5 | 65.5 | 70.iii | 2.2 | 27.5 |
| No family history (n = 10,454) | |||||||
| fifty–64 y | 2,964/ii,805 | fourteen.5 | 3.1 | 82.4 | 52.half-dozen | iv.4 | 43.0 |
| ≥65 y | 2,385/ii,300 | 23.4 | 3.2 | 73.iv | 61.2 | 4.seven | 34.1 |
| No response (n = 13,140) | |||||||
| 50–64 y | three,914/three,412 | 9.2 | 1.iv | 89.3 | 34.iv | 3.8 | 61.8 |
| ≥65 y | iii,122/2,692 | xv.3 | 1.8 | 82.eight | twoscore.9 | 3.ane | 56.0 |
Abbreviations: CT–C, computed tomography colonography; FOBT, fecal occult blood test; FS, flexible sigmoidoscopy.
a Family history defined as self-reporting as a first-caste relative (biological parent, sibling, or child) of a CRC patient.
b Full respondents in the category in 2005 and 2010, respectively.
 Tabular array iii. Adjusted Likelihood of Colonoscopy Among United states of america Adults Aged 40 or Older With a Family unit History of Colorectal Cancer (CRC)a, National Health Interview Survey 2005 and 2010 (n = 26,064)b
Tabular array iii. Adjusted Likelihood of Colonoscopy Among United states of america Adults Aged 40 or Older With a Family unit History of Colorectal Cancer (CRC)a, National Health Interview Survey 2005 and 2010 (n = 26,064)b
| Category | Colonoscopy (vs No Colonoscopy), Odds Ratio (95% Conviction Interval) |
|---|---|
| Year | |
| 2005 | i.0 [Reference] |
| 2010 | v.4 (v.0–5.viii) |
| Sex | |
| Male | 1.0 (0.9–1.i) |
| Female | 1.0 [Reference] |
| Age | |
| 40–49b | 1.0 [Reference] |
| 50–64 | 2.6 (2.0–three.3) |
| ≥65 | iii.6 (ii.7–4.seven) |
| Race/ethnicity | |
| White | 1.0 [Reference] |
| African American | 1.0 (0.ix–1.1) |
| Hispanic | 0.8 (0.7–1.0) |
| Other | 0.seven (0.half dozen–0.viii) |
| Family history of CRC | |
| Have a family history | i.7 (1.5–one.9) |
| No family history | 1.0 [Reference] |
| No response | 0.5 (0.5–0.6) |
| Insurance | |
| Private | iii.iii (two.8–3.9) |
| Public | iii.4 (two.eight–four.ane) |
| Uninsured | 1.0 [Reference] |
a Family history defined as cocky-reporting every bit a start-degree relative (biological parent, sibling, or kid) of a CRC patient.
b Additionally adjusted for marital status and instruction. Income was non significant and was excluded from the model.
Top
Appendix. National Health Interview Survey (NHIS) Questions on Colorectal Cancer Family unit History and Screening Tests, 2005 and 2010
The survey questions on colorectal cancer family history and screening are located in the sample adults cancer file. Questions are similar in both years. The original questions used to create the study variables are the following:
| Question | Answer |
|---|---|
| What kind of cancer did your father have . . . colon? | 1, Mentioned; 2, Non mentioned; 7, Refused; eight, Not ascertained; 9, Don't know |
| What kind of cancer did your father have . . . rectum? | |
| What kind of cancer did your mother accept . . . colon? | |
| What kind of cancer did your mother take . . . rectum? | |
| What kind of cancer did your (brother/brothers) accept . . . colon? | |
| What kind of cancer did your (brother/brothers) have . . . rectum? | |
| What kind of cancer did your (brother/brothers) have . . . rectum? | |
| What kind of cancer did your (sister/sisters) have . . . colon? | |
| What kind of cancer did your (sister/sisters) take . . . rectum? | |
| What kind of cancer did your (son/sons) have . . . colon? | |
| What kind of cancer did your (son/sons) accept . . . rectum? | |
| What kind of cancer did your (daughter/daughters) have . . . colon? | |
| What kind of cancer did your (daughter/daughters) have . . . rectum? | |
| NHIS 2010 | |
| Nigh recent sigmoidoscopy, fourth dimension categories (using 2005 method) | 1, A year ago or less; ii, More than 1 year only not more than 2 years; 3, More than ii years simply not more than than 3 years; iv, More than than 3 years but not more than 5 years; v, More five years only not more than 10 years; 6, Over 10 years ago; 7, Refused; 8, Non ascertained; 9, Don't know |
| Nigh recent dwelling house blood stool test, fourth dimension categories (using 2005 method) | |
| About contempo CT colonography or virtual colonoscopy, time categories | |
| NHIS 2005 | |
| Was this Almost RECENT exam a sigmoidoscopy, colonoscopy, proctoscopy or something else? | 1, Sigmoidoscopy; ii, Colonoscopy; 3, Proctoscopy; iv, Something else; 7, Refused; 8, Not ascertained; 9, Don't know |
| Most recent colorectal test, fourth dimension categories | i, A year agone or less; two, More than one year but not more than than 2 years; three, More than than two years but not more than than 3 years; 4, More than 3 years only not more than 5 years; 5, More than than v years but non more than 10 years; 6, Over x years ago; 7, Refused; viii, Not ascertained; 9, Don't know |
| Most recent part blood stool test, time categories | |
Elevation
Source: https://www.cdc.gov/pcd/issues/2015/14_0533.htm
0 Response to "Florida Blue Coverage on Colonoscopy With Family History"
Post a Comment